The use of makeup has been around since virtually the beginning of human history. First recorded as early as 4000 BCE, the ancient Egyptians used makeup, eyeliner in particular, to display wealth and appeal to their gods. Makeup continued to be used around the world in diverse tribal makeup, the signature Geisha makeup, and the embrace of cosmetics in the Renaissance era.
As knowledge and technology advanced, the importance of non-toxic cosmetic products also increased. The negative health effects of cosmetic additives were soon realised, and cosmetic testing practices were implemented by manufacturers. In order to mitigate human suffering, animals were chosen to be the primary test subjects for new cosmetic ingredients and finished products.
In the 21st century, makeup is still used by billions of people each day. In 2019, the global cosmetics industry was valued at US$532 billion and is expected to grow by between 5-7% compound-annual-growth-rate (CAGR) to reach or exceed US$800 billion by 2025. In recent years the ethics of using animals to test cosmetic products are being called into question, including mounting pressure around the world calling for the end of animal testing in the cosmetic industry.
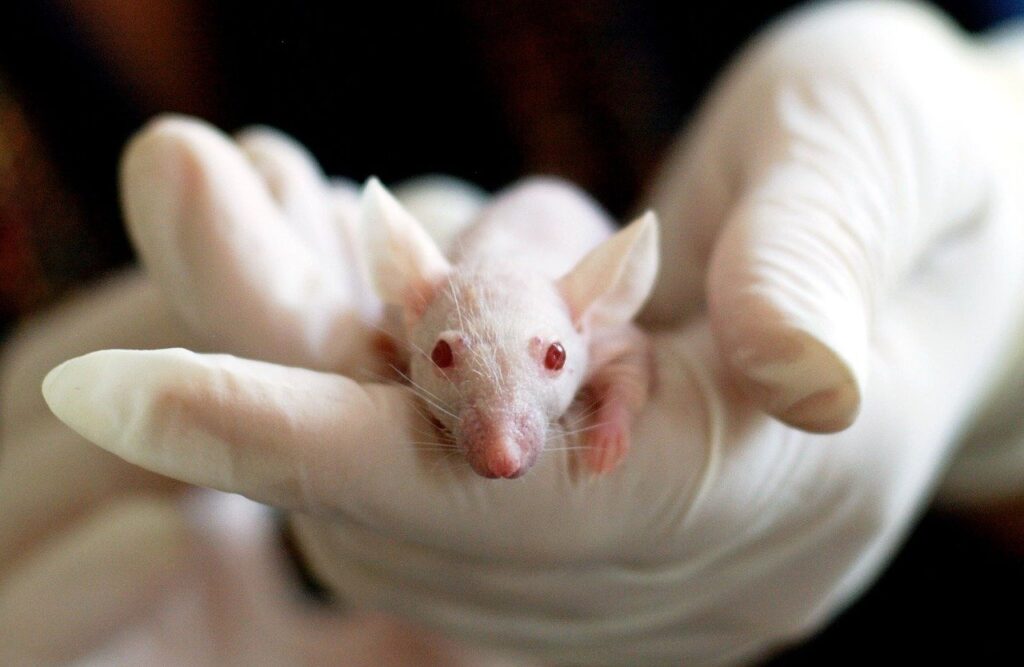

In 2013, the European Union (EU) implemented a ban that prohibits the testing of cosmetic products and ingredients on animals. The EU legislation also included a marketing ban that prohibits the advertisement of cosmetic products and ingredients that were previously tested on animals. The total ban was partially initiated through pressures from social groups advocating for animal rights and the people of Europe demanding change. As demonstrated by the passion of the European people pushing for legislative change, there is a strong public desire for alternative cosmetic testing methods. In order to stay relevant and comply with increasingly aggressive legislation, cosmetic companies must find alternative ways to test their products.
Bioprinting is a type of additive manufacturing, like 3D printing, where living cells mixed with a protective material are printed into any 3-dimensional shape desired. Its ability to create living 3D models shows great potential as an alternative to animal testing, especially in the cosmetic industry. Using lab-grown skin cells, 3D skin samples act virtually the same as human skin would when exposed to cosmetic products, making it a reliable alternative to animal testing. Bioprinted skin samples also have the potential to be a more effective testing method than animal testing since bioprinted skin behaves more closely to human skin than animal skin.
Support from federal governments for the development of alternative testing strategies for cosmetic products opens many avenues for increased bioprinting research and collaboration with cosmetic companies. This support can help to expand the use of bioprinting tissues for cosmetic testing, which include developing tissue samples with varying physical and chemical characteristics. Skin can differ in many ways between populations and should be accounted for when testing cosmetic products. Bioprinting allows for these differences to be taken into consideration and well replicated.

The use of bioprinting for these applications will provide the most scientifically relevant data when superior materials are used. GelMA, one of the most tunable hydrogels, makes an excellent candidate for bioprinting skin because it can facilitate precise control of melanin presence, protein concentration, skin thickness and age; all characteristics that can be altered to simulate different ethnicities, skin makeup, conditions and aging. These varying characteristics create a diverse research sector which allows many different research groups to use the same, superior hydrogel, GelMA, to conduct varying research in different environments without overlap.
The future is clear: animal testing is no longer a tolerated method of testing cosmetic products, however cosmetics must still be tested for human safety. The cosmetic industry is diverse and growing, therefore cosmetic companies will need to start outsourcing for alternative testing methods. The opportunity for bioprinting research to be incorporated into cosmetic company operations will come, and the commercialization of bioprinted skin tissues will follow suit.
The GelMA Company specializes in making the GelMA hydrogel the most accessible hydrogel in the world. In this way, more researchers will be able to use GelMA to advance bioprinting technology and create a world free of animal testing.
Sources
Danzinger, P. N. (2019). 6 Trends Shaping The Future Of The $532B Beauty Business. Forbes.
Senate of the United States (2017). S. 1113, To amend the Federal Food, Drug, and Cosmetic Act to ensure the safety of cosmetics. 1–103.
European Commission. (2013). Communication From the Commission To the European Parliament and the Council on the animal testing ban and marketing ban. Journal of Chemical Information and Modeling, 53(9), 1689–1699.